
NEPHELOstar Plus
Microplate nephelometer for light-scattering and turbidity measurements
Measuring light scattering is one of the most accurate and expedient ways to determine drug solubility early in the drug discovery process or at other crucial steps along the drug development path. Find out how the NEPHELOstar® Plus can be used for drug solubility screening at high throughput.
 Dr Barry Whyte
Dr Barry Whyte
Today’s drug discovery environment demands efficiency, performance and safety. A crucial part of the process is the ability to perform ADMET (absorption, distribution, metabolism, elimination and toxicity) assays as early as possible in the drug development process. For example, researchers need to know quickly if a drug does not have the right toxicity profile before investing further resources and time in advancing it to preclinical and clinical testing.
Solubility is one of the crucial properties of a drug and significantly impacts not only assay development, drug bioavailability, absorption and toxicity studies but also drug dosing and drug formulation.1-3 Researchers in the life sciences therefore need a rapid cost-effective solution to determine solubility before running more costly tests at later stages of the drug development process.
In this blog, we look at the steps typically involved in drug discovery and how drug solubility studies, nephelometry and microplate readers can help to accelerate the process.
The drug discovery and development process for the life sciences is intricate and time consuming and there is no “one-size-fits-all” solution. Rather there is a necessary sequence of steps with many different options along the way (Fig. 1). 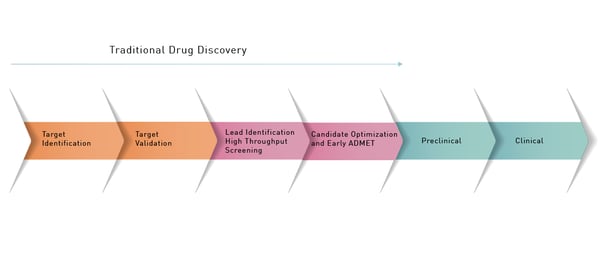 One of the first steps in the drug discovery process is to identify a molecular target that might be an enzyme or other biological molecule that plays a role in a disease process. With this information in hand, researchers can validate the target through different studies. For example, this may involve generating a drug-resistant mutant of the potential target or performing knockdown or overexpression studies of the likely target. Once the target is validated as being of interest the next step is hit discovery.
One of the first steps in the drug discovery process is to identify a molecular target that might be an enzyme or other biological molecule that plays a role in a disease process. With this information in hand, researchers can validate the target through different studies. For example, this may involve generating a drug-resistant mutant of the potential target or performing knockdown or overexpression studies of the likely target. Once the target is validated as being of interest the next step is hit discovery.
High-throughput screening is an important element of this part of the process to screen and identify molecules or chemicals that can interact with the validated drug target.
Typically, compounds are screened to identify binding interactions with the target of interest or modulators of its biological activity. Sometimes virtual screening using computer modelling may be a part of the solution.
After the early steps of testing, only a few compounds or chemicals will warrant further study. The discovery process for a pharmaceutical is more or less complete and the next steps in the development of drug molecules can take place.
Once a potential hit is identified, the molecule is optimized for potency, selectivity and pharmacokinetic properties in a step often referred to as lead optimization.
At this stage, researchers also want to clearly work out the potential benefits and mechanism of action. Dosage experiments for pharmaceuticals will be performed once the best route is known (for example oral or by injection). Other considerations will include how it affects different groups of people and how it might interact with other drugs and treatments.
Once this is over the drug discovery and early development process is complete and the pharmaceutical molecule is ready for extensive preclinical testing in animal model systems (if a suitable one is available). Preclinical development, clinical development, drug approval and post-marketing surveillance mark the next steps in the process to launch a pharmaceutical. A detailed discussion of each of these steps in the context of drug solubility is beyond the scope of this article. But as mentioned earlier there are some later stage processes in clinical development that also benefit from solubility studies. For example, formulation development studies typically occur throughout phase 1 and phase 2 clinical trials. These formulation studies take different factors into consideration including particle size, pH and drug solubility. By phase 3, the formulation used for a pharmaceutical should be close to the one which will used in the approved drug.
Overall, the full journey from drug hit to market approval can take 10-15 years for a new medicine (source: industry group PhRMA) but clearly some types of drugs are exceptions and may be brought to the market in shorter timeframes.
Drug solubility studies are designed to evaluate the solubility of a drug in various solvents or buffers under different conditions. They typically involve measuring the amount of a drug that can be dissolved in a given solvent at a specific temperature or pH. The solubility is often expressed as the maximum concentration of the drug that can be dissolved in the solvent which is also known as the saturation concentration.
Drug solubility tests are essential at different steps along the drug discovery pathway. Out of all the criteria used in early chemical screening poor solubility tops the list of the most undesirable properties. This reflects the fact that molecules with low solubility carry a high risk of failure. Accordingly, it is desirable to test solubility as early as possible in the drug discovery process.
Poor solubility is not only a barrier to testing the activity of a new drug. It also may instigate other undesirable outcomes including influence on other assays, hiding other undesirable characteristics, and potential impact on the kinetic and dynamic properties of a drug. Overall, this can lead to significant delays in drug development times or failure in the development path before suitable refinements are even attempted.
Typically, equilibrium solubility assays have been determined with limited throughput by shaking the drug and target together at a constant temperature for at least 24 hours and measuring the concentration of drug in solution (shake-flask method). The final concentration is often determined by high-performance liquid chromatography but this overall process is time consuming and lacks throughput. Kinetic solubility assays offer higher throughput compared to equilibrium testing and techniques like nephelometry can be readily adapted to a microplate format for this type of assay. This allows solubility screens to be performed much earlier in the drug discovery process and accordingly decreases the risk of late failure and its associated costs.
Nephelometry is an attractive method to study drug solubility because it is a fast, scalable, sensitive and accurate way to measure the concentration of particulate matter in a sample. In addition, it is a non-destructive technique that requires little sample preparation and is readily adapted to a high-throughput microplate format.
The NEPHELOstar Plus from BMG LABTECH is a dedicated microplate nephelometer that detects insoluble particles in liquid samples by measuring forward scattered light. This method relies on measuring the intensity of the light scattered by insoluble particles in the sample. The high-intensity light source of the NEPHELOstar Plus is a laser at 635 nm. This laser beam passes through the sample well into a scattered light detector known as an Ulbricht sphere. Light passes straight through the sphere and no signal is generated if it is not deflected by particles. However, light is scattered and reflected around the interior of the sphere and detected by a photodiode if particles are present in the sample. Light scattered by up to 80 degrees is collected by the Ulbricht sphere (Fig. 2). 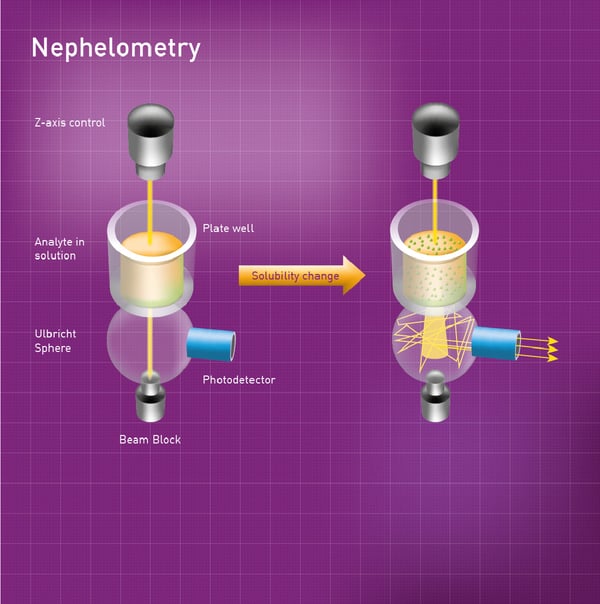 The application note “A fully automated kinetic solubility screen in 384-well plate format using nephelometry” describes how light scattering can be used to measure drug solubility on the NEPHELOstar Plus and gives a good sense of how this method can be used to look at the solubility of different drugs at scale. Overall, the kinetic solubility of 24 compounds was determined in 75 minutes on a 384-well microplate format. The screen was used to measure and rank the kinetic solubility of approximately 90% of discovery compounds submitted to the screening process.
The application note “A fully automated kinetic solubility screen in 384-well plate format using nephelometry” describes how light scattering can be used to measure drug solubility on the NEPHELOstar Plus and gives a good sense of how this method can be used to look at the solubility of different drugs at scale. Overall, the kinetic solubility of 24 compounds was determined in 75 minutes on a 384-well microplate format. The screen was used to measure and rank the kinetic solubility of approximately 90% of discovery compounds submitted to the screening process.
Serial dilutions of each compound were prepared in solution to perform the screen. The levels of particulates at each concentration were detected by light scattering with a microplate nephelometer. Graphs of drug concentration versus light scattering counts were plotted for each drug tested. In most cases, the graphs show a dramatic increase in counts which corresponds to the compound precipitating out of solution. Two linear lines are fitted to the data and the point of intersection corresponds to the kinetic solubility. If the compound is fully soluble at all concentrations, there is only one line on the graph.
A typical kinetic solubility curve for the drug Dofetilide is shown in Fig. 3. Dofetilide is used to correct the irregular heartbeat of patients with atrial fibrillation or to adjust atrial flutter to a normal heart rhythm. At 0.6 mM Dofetilide, the solubility was determined to be 132 µg/ml from the intersection of the two straight lines. This type of analysis quickly identifies at which concentration drug solubility might become an issue for a specific drug.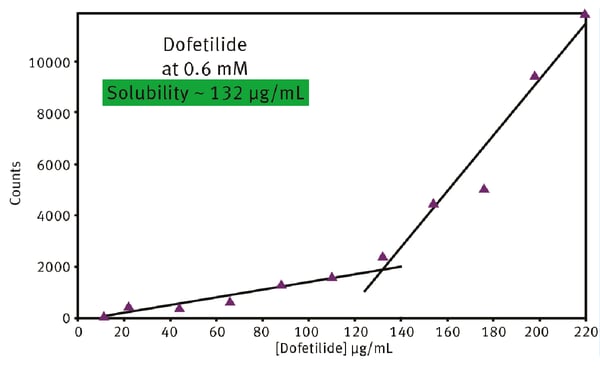 The activity of a new drug will be affected by many factors beyond concentration. This includes the pH, temperature and the presence of other chemical constituents in the body fluid or tissue where the drug needs to access. Bioavailability is therefore impacted by drug solubility under different conditions and it helps to have a technique like nephelometry to rapidly probe the likely effects of these different conditions on the bioavailability of a drug under development.
The activity of a new drug will be affected by many factors beyond concentration. This includes the pH, temperature and the presence of other chemical constituents in the body fluid or tissue where the drug needs to access. Bioavailability is therefore impacted by drug solubility under different conditions and it helps to have a technique like nephelometry to rapidly probe the likely effects of these different conditions on the bioavailability of a drug under development.
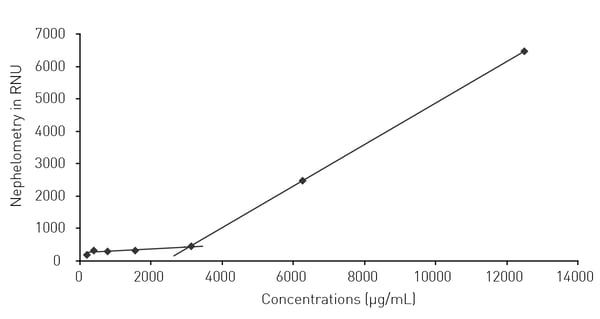
In the application note Nephelometric monitoring growth of Candida albicans using BMG LABTECH’s NEPHELOstar® Plus laser nephelometry was shown to be a reliable method for the measurement of the drug solubility for two antifungal agents. Econazole-nitrate and ciclopirox-olamine were investigated to ascertain their effects on the growth of C. albicans in the presence and absence of cyclodextrin. Cyclodextrins are known to enhance the solubilities of certain compounds leading to increased water solubility, enhanced bioavailability, improved stability and reduced side effects. The solubility diagram for the cyclodextrin-Econazole-nitrate complex is shown in Fig. 4. In this case, cyclodextrin increased the solubility up to 3.1 mg/ml.
 Cancer is a good example of a therapeutic area where drug development and treatment benefits from early-stage drug solubility studies. Cancer patients need access to different drugs in many cases with improved efficacy. Disease recurrence is also significant for many cancer patients. More drugs are therefore needed to meet the needs of patients and the pace of development must accelerate without compromising patient safety.
Cancer is a good example of a therapeutic area where drug development and treatment benefits from early-stage drug solubility studies. Cancer patients need access to different drugs in many cases with improved efficacy. Disease recurrence is also significant for many cancer patients. More drugs are therefore needed to meet the needs of patients and the pace of development must accelerate without compromising patient safety.
In the research report Hyperthermia improves solubility of intravesical chemotherapeutic agents researchers looked at the solubility profiles of three intravesical chemotherapy agents under different conditions.4
The drugs mitomycin C, gemcitabine and cisplatin are commonly used to treat bladder cancer but factors contributing to their bioavailability under different conditions of treatment needed to be established. In some treatments for bladder cancer, drugs are delivered at elevated temperatures (hyperthermia) and it is important to know the effects of these conditions on individual drugs or different combinations of molecules.
In this study, nephelometry was used to assess the impact of different conditions on drug solubility. Heat increased the solubility of all three drugs tested but pH largely had little effect except for a slight reduction in solubility for gemcitabine at pH 8. Mitomycin C at the commonly used concentration of 2.0 mg/ml was insoluble at room temperature but soluble at both 37 and 43º C. Useful information on the bioavailability of each drug was obtained.
High-throughput solubility screens performed early in the drug development process that mimic the conditions of clinical treatment can therefore help considerably in identifying viable drug candidates since insoluble compounds are unlikely to achieve sufficient bioavailability.
Overall, information from high-throughput drug solubility studies helps inform decision making – this prevents delays and helps save costs.
What detection options are available to researchers in the life sciences who want to use nephelometry to look at drug solubility?
The NEPHELOstar Plus from BMG LABTECH is the microplate reader of choice for measuring light scattering and is the ideal partner for drug solubility studies. As the world’s first and only laser-based microplate nephelometer it was developed to meet the high-throughput requirements for fast compound solubility screens.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities and the NEPHELOstar Plus is no exception. By combining high performance with miniaturized assays and short measurement times, the NEPHELOstar Plus offers considerable savings on time, materials and other resources.
Microplate nephelometer for light-scattering and turbidity measurements
Life in the depths of the ocean operates under extreme conditions. Find out how proteins from deep-sea luminescent organisms are useful for measurements on microplate readers.
Next generation sequencing (NGS) technologies for DNA or RNA have made tremendous progress in recent years. Find out how microplate readers can advance the quality control of nucleic acids to facilitate NGS.
Mitochondrial toxicity can have devastating effects on the cell and life. Find out how microplate readers can be used to assess mitochondrial health and how this impacts disease and drug discovery.
Find out how microplate readers can be used to measure histone deacetylase (HDAC) activity and assist drug discovery.
Read how various nucleic acid quantification methods, ranging from UV absorbance and fluorescent dyes to amplification methods, can be performed on BMG LABTECH microplate readers.